Top 7 Things You're Forgetting When Reprocessing Your Ultrasound Probe

Maintaining infection prevention & control (IPC) protocol for your ultrasound probes that ensures the integrity of your transducers and the safety of your patients is important to work. It also, unfortunately, can be trickier than it looks.
In a recent systematic review,1 Dr. Nancy Moreau and Dr. Gregory E. Gilbert evaluated seven individual studies, each focusing on infection outbreaks within the ultrasound suite. Throughout their research, they determined that each of the outbreaks examined was the direct result of failures in the facilities' probe decontamination process. Even worse, they found that these failures were all preventable.
Their study is far from the first to document the danger of infection inherent to deficient probe decontamination processes - even a cursory look through the medical literature reveals example 2 after example 3 after example 4 of just how dangerous - and rampant - a contamination threat improper probe IPC can pose to the safety of the ultrasound suite if left unchecked.
It's hard to understate the importance of maintaining a proper IPC protocol for your ultrasound probes. At CIVCO, we understand what it takes to ensure that your probes remain clear of bacteria and other dangerous pathogens...and we also know what areas tend to be forgotten regarding probe disinfection. Below are 7 ideas for beefing up your probe disinfection process and keeping your probes - and your patients - safe from contamination.

#1: Clean your transducer after examination:
It might seem obvious, but cleaning your transducers after each procedure is key to reducing infections within the ultrasound suite. Always remember to thoroughly remove all gel and residues from the probe after examinations, using soap and water to rinse the device, or applying low-level disinfectants like quaternary ammonium - which can often be found in many germicidal wipes - to clean the transducer and cable after use 5 (consider the Endozime Sponge for TEE Probes, which are uniquely contoured to fit around the outside sheath of scopes and are pre-saturated with Endozime Bio-Clean to provide a quicker, more thorough pre-cleaning). Another good tip? Use a small brush to clean crevices and areas of angulation on your probe, preferably with a nonabrasive detergent - remember that bacteria can easily take root in even the tiniest of spaces, which is why it's important to thoroughly clean every inch of the transducer, including in those areas that are harder to reach. 6

#2: Perform high-level disinfection:
According to the Spaulding Classification System, transducers that are classified as being 'semi-critical' devices (i.e. any instrument that contacts mucous membranes or nonintact skin ) are required to undergo high-level disinfection,7 a chemical process that sees the complete elimination of all microorganisms in or on an instrument, except for a small number of bacterial spores, with hydrogen peroxide, OPA, glutaraldehyde, and peracetic acid all serving as effective HLD solutions that can be used to disinfect your ultrasound probes post-procedure 8. When conducting high-level disinfection, remember to soak the transducer in a high-level disinfectant for 10-20 minutes (depending on the disinfectant used). 9 (Or, if you're short on time or reprocessing a high volume of transducers, let our ASTRA® Automated Disinfection System do the work for you. Compatible with multiple reusable high-level disinfectants (including UltrOxTM (hydrogen peroxide), Revital-Ox® RESERT® (hydrogen peroxide), CIDEX® OPA, and MetriCide™ OPA Plus). ASTRA is designed to streamline your disinfection process, automatically disinfecting and reprocessing up to 2 probes at once in 10-16 minutes. Learn more about how ASTRA can expedite your probe disinfection here.

#3: Always wear PPE:
High-level disinfectants can result in adverse health effects, including dermatitis, mucous membrane irritation, skin and respiratory tract irritation, and asthma-like symptoms such as wheezing and shortness of breath. 10 As a result, it is crucial that healthcare workers who perform manual high-level disinfection protect themselves, including wearing proper protective personal equipment (PPE) such as water-resistant gowns, protective gloves/eyewear, and/or face protection.11 To ensure even further protection when performing high-level disinfection, healthcare workers should consider utilizing either a manual disinfection station with proper ventilation controls, such as the GUS Disinfection Soak Systems, or an automated probe reprocessor, such as the ASTRA Automated Ultrasound Probe Disinfection System, when disinfecting transducers.
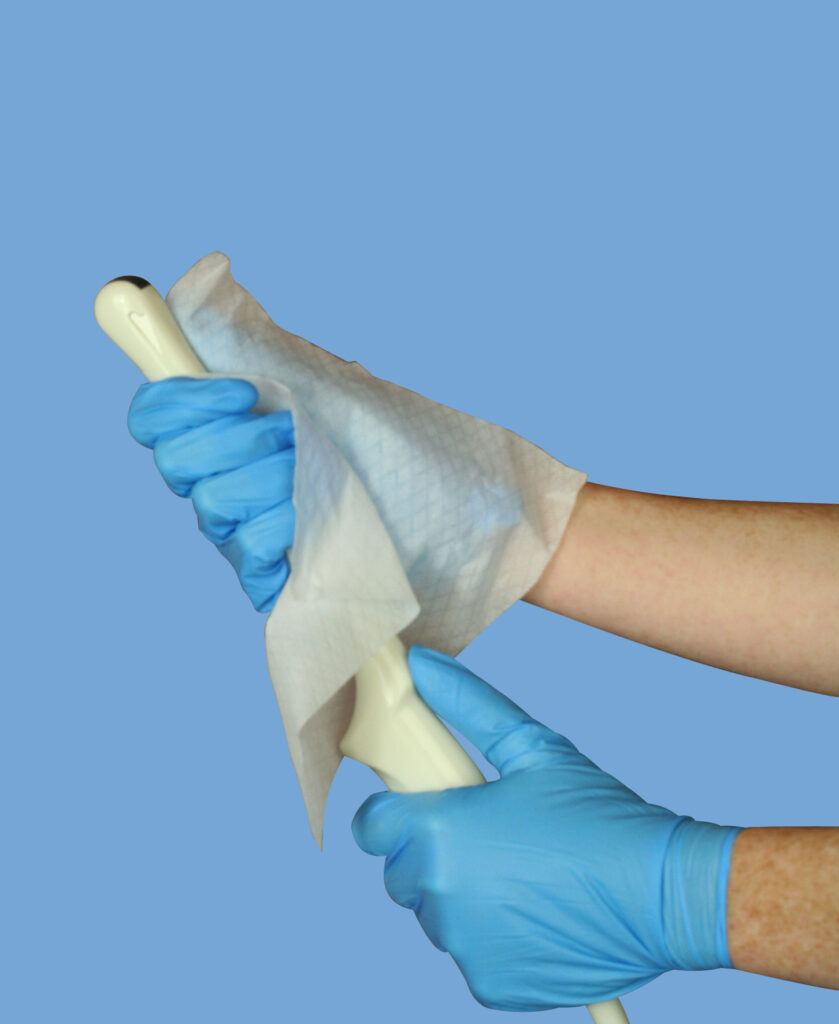
#4: Dry the transducer after cleaning:
Just as ultrasound gel that is left sitting on the probe can increase the chances of possible contamination, water that is left undried on the probe post-cleaning can also negatively impact the disinfection process, diluting the disinfectant and lessening the overall effectiveness of the solution. Remember to use a cloth to completely wipe and dry the transducer and cable after each cleaning session - it takes only a few seconds to do but can have a substantial impact in ensuring that your high-level disinfectant decontaminates your probe as thoroughly as possible. 6
#5: Thoroughly inspect your TEE probe:
Make a habit of visually inspecting your probe after cleaning to ensure cleanliness, while also keeping an eye out for possible holes or defects in the body of the transducer. It's important to remember that bacteria can often reside in even the smallest of spaces on your probe, which is why it's crucial to inspect the entire body of the transducer - including dents and crevices - for any remaining gel, soil, or bioburden (and when necessary, don't hesitate to replace damaged probes, as well). 12

#6: Store TEE probes appropriately, post-procedure:
Properly storing your ultrasound probes after reprocessing can be a great way of making sure that your transducer remains safe from additional contamination after a procedure. In order to protect transducers from potentially harmful pathogens, consider a probe storage cabinet that is equipped with shelves that separate the high-level disinfected from the low-level disinfected parts of the transducer, as well as proper filtration. (To read more about the effectiveness of hanging times when stored in a positive pressure HEPA-filtered cabinet post-HLD on probe safety, check out our Pub Brief on a recently published study.)
CIVCO's Ultrasound Probe Storage System provides a safe and clean storage environment for your disinfected ultrasound probes, complete with a HEPA filtration system that provides continuous airflow using positive pressure, as well as a locking door for added security for your delicate transducers. To learn more, click here.
#7: Centralize your probe reprocessing:
Reprocessing your semi-critical devices is an intricate process and one that oftentimes requires an environment that is free from distraction. Centralizing your TEE probe reprocessing can be helpful not only in shielding medical staff from additional interruptions and ensuring completion of all reprocessing steps but also in allowing for controlled work processes that provide a greater feeling of security to staff and patients during intraoperative procedures. 1
References:
- Souza, H. K., Quartim, . M. B. C., & Uchikawa, G. K. (January 01, 2022). Infection Transmission Associated With Contaminated Ultrasound Probes: A Systematic Review. Aorn Journal, 115, 1, 42-51. https://pubmed.ncbi.nlm.nih.gov/34958475/
- Nyhsen, C. M., Humphreys, H., Nicolau, C., Mostbeck, G., & Claudon, M. (December 01, 2016). Infection prevention and ultrasound probe decontamination practices in Europe: a survey of the European Society of Radiology. Insights into Imaging, 7, 6, 841-847. https://pubmed.ncbi.nlm.nih.gov/27778309/
- Sartoretti, T., Sartoretti, E., Bucher, C., Doert, A., Binkert, C., Hergan, K., Meissnitzer, M., ... Gutzeit, A. (October 01, 2017). Bacterial contamination of ultrasound probes in different radiological institutions before and after specific hygiene training: do we have a general hygienical problem?. European Radiology, 27, 10, 4181-4187. https://pubmed.ncbi.nlm.nih.gov/28374081/
- M'Zali, F., Bounizra, C., Leroy, S., Mekki, Y., Quentin-Noury, C., & Kann, M. (January 01, 2014). Persistence of microbial contamination on transvaginal ultrasound probes despite low-level disinfection procedure. Plos One, 9, 4.) https://pubmed.ncbi.nlm.nih.gov/24695371/
- Shokoohi, H., Armstrong, P., & Tansek, R. (January 01, 2015). Emergency department ultrasound probe infection control: challenges and solutions. Open Access Emergency Medicine : Oaem, 7, 1-9.
- American Institute of Ultrasound Medicine (AIUM), "AIUM Official Statements - Guidelines for Cleaning and Preparing External- and Internal-Use Ultrasound Transducers and Equipment Between Patients as well as Safe Handling and Use of Ultrasound Coupling Gel," 2017. https://www.aium.org/officialstatements/57
- Centers for Disease Control (CDC), "A Rational Approach to Disinfection and Sterilization. Guideline for Disinfection and Sterilization in Healthcare Facilities," 2008. https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf
- Centers for Disease Control (CDC), "Guideline for Disinfection and Sterilization in Healthcare Facilities - Comparison of the characteristics of selected chemicals used as high-level disinfectants or chemical sterilants," 2008. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/tables/table4.html
- Vickery, K., Gorgis, V. Z., Burdach, J., & Patel, D. (January 01, 2014). Evaluation of an automated high-level disinfection technology for ultrasound transducers. Journal of Infection and Public Health, 7, 2, 153-60. https://pubmed.ncbi.nlm.nih.gov/24314741/
- Centers for Disease Control (CDC), "Health and Safety Practices Survey of Healthcare Workers - High-Level Disinfectants," 2018. https://www.cdc.gov/niosh/topics/healthcarehsps/disinfect.html
- Society of Gastroenterology Nurses and Associates, Inc. (SGNA), "Guideline for Use of High-Level Disinfectants & Sterilants in the Gastroenterology Setting," 2017. https://www.sgna.org/Portals/0/HLD__FINAL.pdf
- Society of Diagnostic Medical Sonography (SDMS), "Guidelines for Infection Prevention and Control in Sonography: Reprocessing the Ultrasound Transducer," 2020. https://www.sdms.org/docs/default-source/Resources/8756479320933256.pdf







